BRIGHTSCAN™ THERMOGRAPHIC DISPLAY SYSTEM 2G
TOUCH-FREE TEMPERATURE MEASUREMENT WITH FACIAL RECOGNITION AND MASK DETECTION CAPABILITIES
The BrightScan™ Thermographic Display System 2G (BTDS-2) is a new and advanced AI (Artificial Intelligence) model with face recognition and thermal scanner system capabilities. It accurately identifies a person and measures their body temperature, with or without a mask or glasses. Perfect for offices, hotels, healthcare (adjunctive diagnostic screening only), schools, restaurants, retail, transportation, and other public spaces.
Features
- 8.0-inch IPS LCD display
- Integrated camera, thermal sensor and display
- Touchless body temperature
- Mask detection for entry
- Facial recognition for point of entry
- Built for 24/7 operations
- Integrate with existing access control systems
- Visitor log management
- Table stand, floor stand or wall mount options
- Custom screen saver
- Custom voice message

Records body temperature with ≤ 0.5°F accuracy in under one second with audible feedback


Keeps track of name, date, time and temperature of entrants

***Be Wary of Fly-By-Night Companies***
There are several companies in Hawaii that were just started in the past few months just to take advantage of our current COVID situation. Make sure to buy only from reputable companies that have been around for a while and will stand behind their product. Bright Light Digital is a Hawaii-based company since 2016. We provide support with every sale and a one-year warranty (at no charge) for our BrightScan thermal scanner. Extended warranties are also available. Mahalo!
Product Details
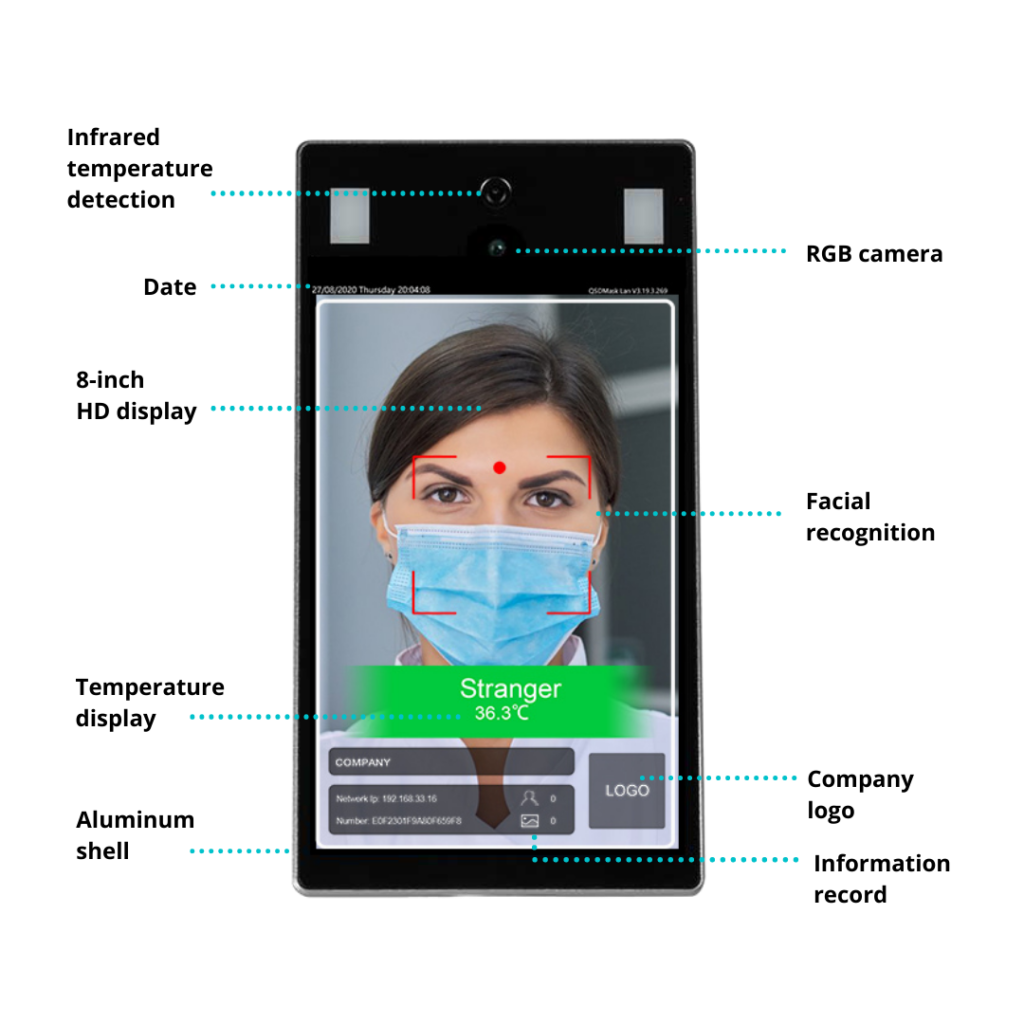
Product Specifications
- Resolution: 1920×1080
- Type: Monocular camera, color
- Aperture: F2.0
- Focusing distance: 0.443 cm
- Dynamic range: ≥120dB
- Field Angle: 75°
- Distortion: <1.5%
- Size: 8.0 inch LCD screen
- Resolution: 1280×800
- Tempered Glass
- Touch: Not supported
- CPU: Android RK3288 quad-core ARM-A17, 1.8GHz
- GPU: Mali-T764
- RAM: DDR 2GB
- ROM: EMMC 8G
- OS: Android 7.1.2
- Network Support: Ethernet, WiFi, BT-4.1
- Speaker: 2W
- USB: 1 USB OTG, 1 USB HOST standard A port
- Relay output: 1 door open signal output
- Wiegand: Output*1 (RS232 ), input*1
- Wired network: 1 RJ45 Ethernet socket
- Credit card reader: None
- Face library: Up to 50,000 images
- Stranger detection: Supported
- Identity distance configuration: Supported
- UI interface configuration: Supported
- Upgrade remotely: Supported
- Interface: Interfaces include device management, personnel/photo management, record query, etc.
- Deployment method: Supports public cloud deployment, privatized deployment, LAN use, stand-alone use
- Sensor: Melexis (Belgium)
- Temperature detection: Supported
- Temperature detection distance: 20cm-60cm
- Temperature measurement accuracy: ≤ ±0.5°F
- Temperature measurement range: 86°~113°F
- Visitors’ temperature is normal and released directly: Supported
- Visitor’s temperature is abnormal: Supported (temperature alarm value can be set)
- Power: DC12V*2
- Operating temperature: 32°F~122°F
- Storage temperature: -4°F~140°F
- Power consumption: 10W
- Installation method: Gate bracket installation
- Size: 9.0″ x 4.7″ x 1.0″ (device only)
Companies and Organizations that have Invested in BrightScan™






























Interested in a BrightScan™ product demonstration?
Call, email or complete our online form to schedule an appointment!
BrightScan™ is not intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, including COVID-19. An indication of an elevated body temperature should be confirmed by a secondary evaluation method, such as a non-contact infrared thermometer or clinical-grade contact thermometer. Various environmental and methodological factors can impact thermal imaging results. This is not an FDA-approved device. A guidance document from the FDA can be viewed here. Proper use of this device is the responsibility of the end user, who must confirm compliance with applicable data privacy, medical data privacy, and employment laws and regulations, including HIPAA compliance.
